- By junaid
- Uncategorized
- 0 Comment
What Is Impressed Current Cathodic Protection (ICCP)?
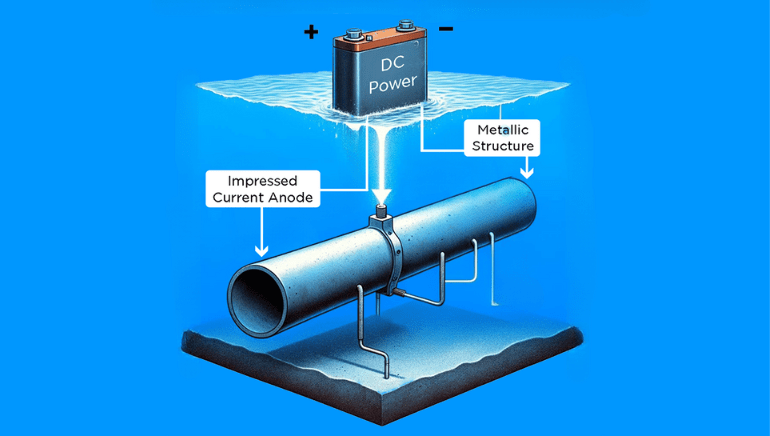
Impressed Current Cathodic Protection (ICCP) is an advanced corrosion prevention technique that utilizes an external power source to provide a continuous electrical current to metal structures. This process actively mitigates the electrochemical reactions leading to corrosion, offering superior control and adaptability compared to traditional methods.Basic Types of Cathodic Protection
There are two fundamental types of cathodic protection: galvanic and impressed current.Galvanic Cathodic Protection
In galvanic cathodic protection, metals with different electrochemical potentials are connected, creating a natural electric current that flows from the more active metal (anode) to the less active metal (cathode), thereby protecting the cathode from corrosion. However, this method has limitations, especially when applied to large or complex structures.Impressed Current Cathodic Protection (ICCP)
ICCP, on the other hand, involves an external power source that provides a continuous electrical current to the structure to counteract the corrosive process. This method allows for greater control and adaptability, making it suitable for a wide range of applications.Principles of ICCP
Understanding the fundamental principles of ICCP is crucial for its effective implementation.Electrochemical Reactions
ICCP relies on electrochemical reactions occurring at the metal surface. By applying an impressed current, the metal structure is polarized, shifting the electrochemical potential to a level where corrosion is minimized or halted.Anode and Cathode Reactions
In ICCP systems, sacrificial anodes are not used. Instead, inert anodes, typically made of mixed metal oxide or platinum-coated materials, are employed. At the anode, water is electrolyzed to produce oxygen and hydrogen ions, while at the cathode, the metal surface is protected from corrosion.Components of ICCP Systems
To implement ICCP effectively, various components are essential.Anode Beds
Anode beds consist of the anodes buried in the ground or submerged in water, providing the electrical current necessary for cathodic protection. The design and placement of anode beds are critical to achieving uniform protection across the entire structure.Power Supply
A dedicated power supply, often a transformer-rectifier unit, is used to provide a controlled and continuous electrical current to the structure. The impressed voltage and current output are carefully regulated to ensure optimal protection without causing damage.Monitoring and Control Systems
Sophisticated monitoring systems are employed to assess the performance of the ICCP system. These systems continuously measure parameters such as structure potential, current density, and anode potential, allowing for real-time adjustments and ensuring the system operates within specified parameters.Applications of ICCP
ICCP finds applications in various industries where corrosion poses a significant threat to infrastructure and equipment.Maritime Industry
Ships, offshore platforms, and submerged structures benefit from ICCP to protect against corrosion caused by saltwater exposure.Oil and Gas Pipelines
ICCP is widely used to protect pipelines transporting oil and gas, especially in corrosive environments, preventing leaks and structural deterioration.Infrastructure
Bridges, piers, and other critical infrastructure exposed to harsh environmental conditions often employ ICCP to extend their service life.The Corrosion Problem
In the domain of material longevity, a pervasive adversary looms: corrosion. This natural electrochemical process, commonly identified as rusting, takes hold when metals are exposed to environmental elements. In response to this continual threat, the shield of cathodic protection (CP) emerges as a sophisticated defense mechanism. Cathodic protection is a strategic approach, employing an external electrical current generated from a designated anode. This current permeates the ambient environment, constructing a methodical shield around the metal structure, resolutely opposing the encroachment of corrosion. The effectiveness of cathodic protection lies in its nuanced ability to alter the corrosive environment, effectively halting the progression of this detrimental process. When implemented with meticulous design and application, current CP systems assume the role of silent guardians, preventing corrosion with unwavering efficacy. Cathodic protection is not a universal panacea; it thrives in environments where structures are immersed in conditions conducive to the flow of electrical current. Regrettably, the open air remains impervious to this defense, as air lacks the conductivity required for its application. However, for structures submerged or concealed underground—such as pipelines, ships, docks, and storage tanks—cathodic protection stands as a vital safeguard. In essence, cathodic protection embodies a serious and indispensable facet in the ongoing narrative of metal structures, ensuring their durability and resilience against the relentless corrosion that seeks to compromise their integrity. Through the meticulous deployment of current CP systems, industries fortify their infrastructure, fostering longevity and sustainability in the face of an ever-present threat.Why Impressed Current Cathodic Protection (ICCP)?
ICCP is chosen for its distinct advantages over other cathodic protection methods.Advantages of ICCP
- ICCP significantly extends the lifespan of metal structures by actively preventing corrosion, ensuring they remain structurally sound and functional for an extended period.
- The proactive nature of ICCP minimizes the need for frequent maintenance and repairs, resulting in substantial cost savings over the life of the structure.
- By preventing corrosion-related structural failures, ICCP enhances overall safety, especially in industries where the integrity of metal structures is critical to preventing accidents and environmental damage.
- ICCP systems can be tailored to specific structures and environmental conditions, ensuring optimal protection in diverse applications.
Disadvantages of ICCP
- The installation of ICCP systems involves a higher upfront investment compared to some traditional methods. However, this cost is often justified by the long-term savings in maintenance and repairs.
- ICCP systems can be intricate, requiring careful design and periodic maintenance to ensure continued effectiveness. This complexity demands expertise and diligence in system management.
Conclusion
The Impressed Current Cathodic Protection System (ICCP) stands as a sophisticated and indispensable solution to the pervasive corrosion problem. By understanding its principles, advantages, and considerations, industry professionals can make informed decisions in choosing and implementing ICCP systems. While challenges such as higher initial costs and system complexity exist, the overall impact of ICCP on the durability and safety of metal structures is undeniable. As technology continues to advance, ICCP remains at the forefront of corrosion prevention strategies, ensuring the resilience and longevity of vital infrastructure worldwide.FAQs
ICCP, or Impressed Current Cathodic Protection, is a corrosion prevention technique that utilizes an external power source to provide a controlled electrical current to metal structures, actively halting the corrosion process.
ICCP works by applying a continuous electrical current to the metal structure from an external power source, effectively polarizing the metal and shifting its electrochemical potential to a level that minimizes or halts corrosion.
The Impressed Current technique involves using an external power source, often a transformer-rectifier unit, to deliver a controlled electrical current to a metal structure. This current is crucial for actively preventing corrosion and maintaining the structural integrity of the metal.
Galvanic anode cathodic protection relies on the natural electric current generated by connecting metals with different potentials, while ICCP utilizes an external power source to provide a controlled current. ICCP offers greater control and adaptability, making it suitable for diverse applications compared to the simpler galvanic method.
ICCP is employed to actively prevent corrosion in metal structures by providing a controlled electrical current, ensuring prolonged service life, reduced maintenance costs, and increased safety. It offers superior control and adaptability, making it a preferred choice for various industrial applications.
ICCP typically uses inert anodes, often made of mixed metal oxide or platinum-coated materials. Unlike sacrificial anodes, these inert anodes do not corrode during the protection process, making them suitable for long-term use in ICCP systems.